Understanding pH in Hydroponics
Hydroponics is all about precision. The health of the plants in your growing system depends on various factors – design of your system, crop selection, key parameters such as pH, EC, temperature, humidity among others. These parameters have a range in which they need to be kept in order to get optimal results from the plants.
In most of my training sessions on Hydroponics in India, I have received more questions on pH than any other topic. So i decided to write a series on that.
In this post, we are going to focus on pH. To be precise, pH of the nutrient solution in hydroponics.
So, what is pH?
pH is the short form for potential hydrogen ions. To put it in simple english, pH is a number that indicates the extent to which a solution is acidic or alkaline. pH is measured on a scale of 0-14. The value of 7 being neutral (pure water) and anything less than 7 is acidic and above 7 is alkaline.
pH below 7 indicates more H+ ions(hydrogen ions) and pH above 7 indicates more (OH-) ions(Hydroxyl ions). More H+ means, it is acidic and more OH- is alkaline or basic. Just remember this.
One thing to note here is that the pH scale is logarithmic. It simply means that pH 5 is 10 times more acidic than pH 6 and not just by 1. What this also means is that if 5 ml of acid brought your pH from 7 to 6. Another 5 ml won’t bring it to 5. It might take the pH to 3 or 4 or even lower. Keep this in mind.
How is pH measured
pH can be measured using various means- pH meter, pH test kit, pH test strips.


pH meter– A digital instrument that measures the pH of the solution you dip it in. It’s a good practice to calibrate these meters once per day of operation. As mentioned above, the value of pH ranges between 0 and 14. pH of 0 being the most acidic and pH value of 14 being the most alkaline.pH meters are available in several form factors. I prefer a handheld pH meter because its very convenient to use.
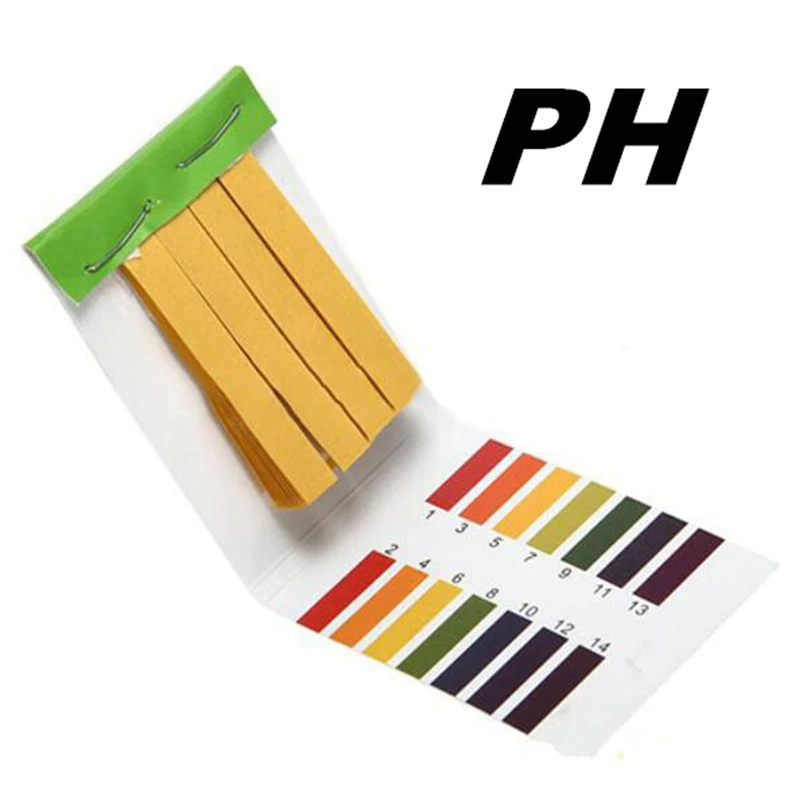
pH test strip – These are paper strips coated with a special compound which when dipped in a solution will change the colour based on the acidity or alkalinity of the solution. Upon comparing the color of the pH strip to the color chart provided with the pH paper kit, one can determine the pH value of the solution. These can be found in aquarium shops.
pH test kit – This test Kit comes with a pH test indicator solution. A few drops of this indicator is added to the sampling solution (in our case hydroponic nutrient solution) and mixed well by shaking. Based on the coloration of the solution and comparing this colour with the pH colour chart provided with the kit, one can determine the pH of the test solution.
Why is pH of the nutrient solution important?
pH of the nutrient solution determines the uptake of nutrients by the plants and the solubility of the nutrient salts.
For e.g if the pH goes higher than the range 5.5-6.5, Iron is less available for plants and due to this, plants will show a deficiency of Iron, though it is present in the solution.
Another common deficiency that can be seen is calcium. Higher pH values result in calcium precipitating in the solution into an insoluble form.
Inexperienced growers may misinterpret this deficiency as absence of the element in the solution and may even add more of those to fix the symptom.
What is the ideal range for pH?
Most plants prefer the nutrient solution to be in the range of 5.5-6.5. The uptake of the nutrient elements with respect to pH is given in the illustration below.
As you can see, from 5.5 to 6.5 the availability of all nutrients are fairly optimal. Drifting either way will result in one or the other essential nutrient left out from being taken up by the plants. So, now you know what is the best pH for hydroponics.
How to adjust pH in Hydroponics
Adjusting pH means, increasing or decreasing the current pH value to the ideal one that is optimal for the plants being grown. Either you bring down the pH to the right value within the range or you bring up the pH.
Remember, the quality of water used for the preparation of the nutrient solutions plays a major role in pH management.
Hard water, due to the presence of carbonates can resist pH changes in the solution. This characteristic is called the alkalinity of the water. This also results in keeping the pH from fluctuating too much from the current value.
Hard water has high buffering capacity due to the presence of carbonates as mentioned above.
Soft water on the other hand, offers very little to no resistance as there are no carbonates to counter the ph change. When using soft water, the pH change will be drastic. Ensure gradual addition of pH Up or pH Down to get the right value.
If you aren’t sure of the water quality that you have, it is better to get the water source tested or go with reverse osmosis water.
Addition of an Acid
pH of a solution comes down on adding an acid. Hence it is called “pH Down” in the hydroponics world. By adding a suitable acid, we can bring down the pH of the nutrient solution to the right range. Now a question arises.
Which acid to use as a pH down solution?
The acids that are fit for usage as pH down are
- Phosphoric acid (Most preferred)
- Nitric acid
- Sulphuric acid
These are strong acids that bring down the pH with small amounts. There are weak acids as well such as acetic acid (vinegar). They aren’t very effective and are not recommended for hydroponics.
Although, technically speaking, any acid should work, there are few considerations for selecting the acid for use as pH down.
- Safe to use by people (Non corrosive, safe to handle)
- Safe for plants (The elements in the acid are safe for plants)
- Economic.
Considering all this , Phosphoric acid is widely used as a pH down soluti0n. Phosphoric acid is available in concentrated form which has to be diluted before using in hydroponic systems.
Usually a 2-5% diluted solution of phosphoric acid is used for pH reduction. Although commercial farms may use a different dilution based on their dosing / controller system capacity.
Additional advantage of using phosphoric acid is that it also supplies the essential nutrient phosphorus to the plants.
If you are into commercial hydroponics farming in India, you may need phosphoric acid in large volumes. Most chemical dealers supply phosphoric acid in 25/35 kg carboys.
Addition of an Alkali/Base
pH of a solution goes up on adding an alkali. Hence it is called “pH Up“. Just like acids, there are certain considerations for selecting pH Up.
Potassium hydroxide is widely used as a pH up solution.
Additional advantage of using potassium hydroxide is that it also supplies the essential nutrient potassium to the plants.
What causes pH of the nutrient solution to fluctuate?
In a hydroponics system that is designed and run well, pH fluctuations are normal. The fluctuations are usually higher when the plants are growing vigorously or the nutrient reservoir size is smaller considering the number of plants.
In such cases, a more frequent adjusting protocol is necessary to maintain or stabilize the pH in hydroponics to the desired value.
Why does pH increase?
As the plants grow, they take up different elements in the nutrients at different rates. During a crop cycle, you might have wondered why my pH is going up. It has been observed that the pH tends to rise in hydroponics during the rapid growth rate of plants.
When the plants grow, they take up nutrients such as Nitrogen (in the form of Nitrate ion NO3). Nitrate is an anion which is negatively charged. During the absorption of an anion, the roots give out another negative charged ion (OH-) to keep the equilibrium of anions and cations in the root zone. This continuous release of OH- ions causes the pH to increase. As we saw in the earlier section, more of OH- results in an alkaline solution.
The plants don’t just take nitrate alone, they take various elements in a ratio suitable for their growth. This is due to the selective absorption of nutrients.
Why does pH decrease?
When plants take up cations in the nutrient solution such as Potassium, Calcium and Magnesium ( K+, Ca2+ and Mg+), the roots balance the equilibrium by releasing a H+ ion. This causes the pH of the nutrient solution to drop causing it to be more acidic.
Based on the direction of the drift-pH rise or pH drop, it is possible to understand what is happening in the rootzone of the plant and if required a corrective action can be taken to maintain pH balance in hydroponics and keep the pH stable.
That brings us to the end of this post and if you made it this far, thank you for your interest.
Wait! There is more
I have made a video series on pH in Hydroponics and I think you will like it.
If you are interested in more such articles related to hydroponics in India, please send in your suggestions and comments.
Happy Farming!





6 Responses
[…] Beginner’s guide to pH in Hydroponics – GEEKGARDENER […]
Absolutely rich. Thank you very much .
Sir, this info so superb. Well explained and appreciate your efforts in making such posts which serves as a Bible to noob folks like me.
Thanks for your nice words. Glad it is helpful
Thank you very for your valuable videos and appreciated.
With regards,
Nissamudeen
K.S.A
Thank you for your nice words